Application
Contact Us
Phone
Address
No. 60, Yungjing East Road, Liyuan Town, Tongzhou District, Beijing City, China
Amination
Category:
Application
The ammoniation process refers to an industrial procedure that converts nitrogen-containing substances into ammonia (NH₃) or amino-containing (-NH₂) compounds through chemical reactions. Its core mechanism involves transforming nitrogen sources (e.g., urea, nitrates, organic nitrogen compounds) into ammonia or combining ammonia with other substances to generate derivatives, typically under high temperature and pressure, catalytic reactions, or biological conversion. This process is a critical component of nitrogen cycle utilization and is widely applied in the chemical industry, agriculture, and environmental protection sectors.
Ammoniation reactions are often conducted under high temperatures (300–500°C) and high pressure (10–30 MPa). Equipment may experience leaks or explosions due to overpressure or extreme heat. Fluctuations in reaction conditions (temperature, pressure, concentration) can trigger uncontrolled "thermal runaway" reactions, potentially leading to chain explosions. Ammonia gas (NH₃) is highly irritating; leaks can cause poisoning, respiratory tract burns, or eye injuries. Byproducts such as carbon monoxide (CO) and hydrogen gas (H₂) may result in asphyxiation or combustion.
While ammoniation technology is a cornerstone of modern chemical systems, its high-risk nature demands strict corporate control over process parameters, equipment integrity, and personnel safety measures.
Technical Trends
Globally, ammoniation processes are evolving toward low-carbon pathways (green hydrogen-based ammonia synthesis), intelligent systems (digital twin monitoring), and resource circularity (ammonia recycling). Regulatory frameworks are increasingly aligning with carbon neutrality goals.
| Country/Region | Regulatory Focus | Core Equipment Technologies |
| United States | Risk management & emission control | Microchannel reactors + SCR denitrification |
| EU | Green processes & modular design | Proton exchange membrane electrolytic reactors + heterogeneous catalysis |
| China | Localization & intelligent upgrades | Multi-stage cascade reactors + integrated ammonia-based desulfurization/denitrification |
| Japan | Seismic resistance & energy efficiency optimization | Seismic-resistant fixed-bed reactors + waste heat power generation |
Case
PMG assisted a client in developing continuous production. The reaction process is divided into two steps: the first involves nucleophilic substitution, and the second neutralizes the acid generated in the first step. A tandem system combining a microchannel reactor and a multiphase reactor was adopted. Based on a first-step residence time of 20 seconds, the total annual throughput is 2,862.2 t/a. With a multiphase reactor holdup volume of 50 L, the reaction time in the tubular reactor was calculated as 358 seconds.

Reaction equation
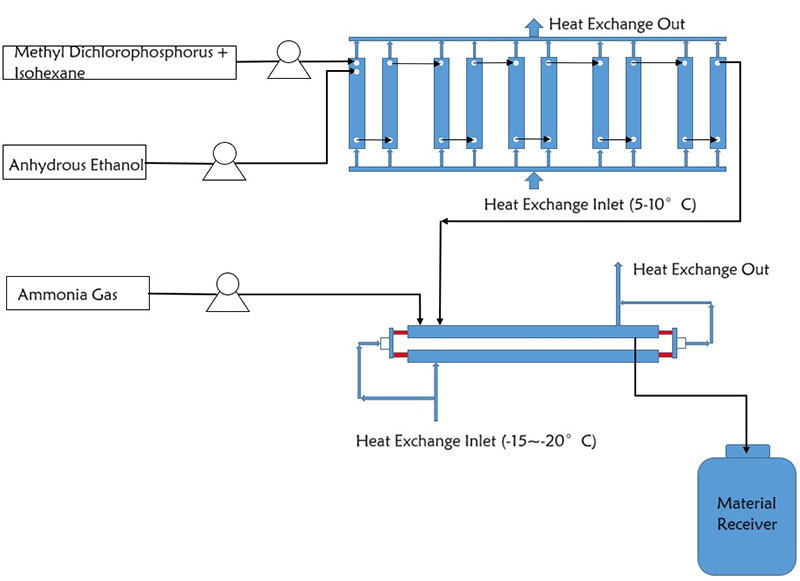
Rocess Flow Diagram
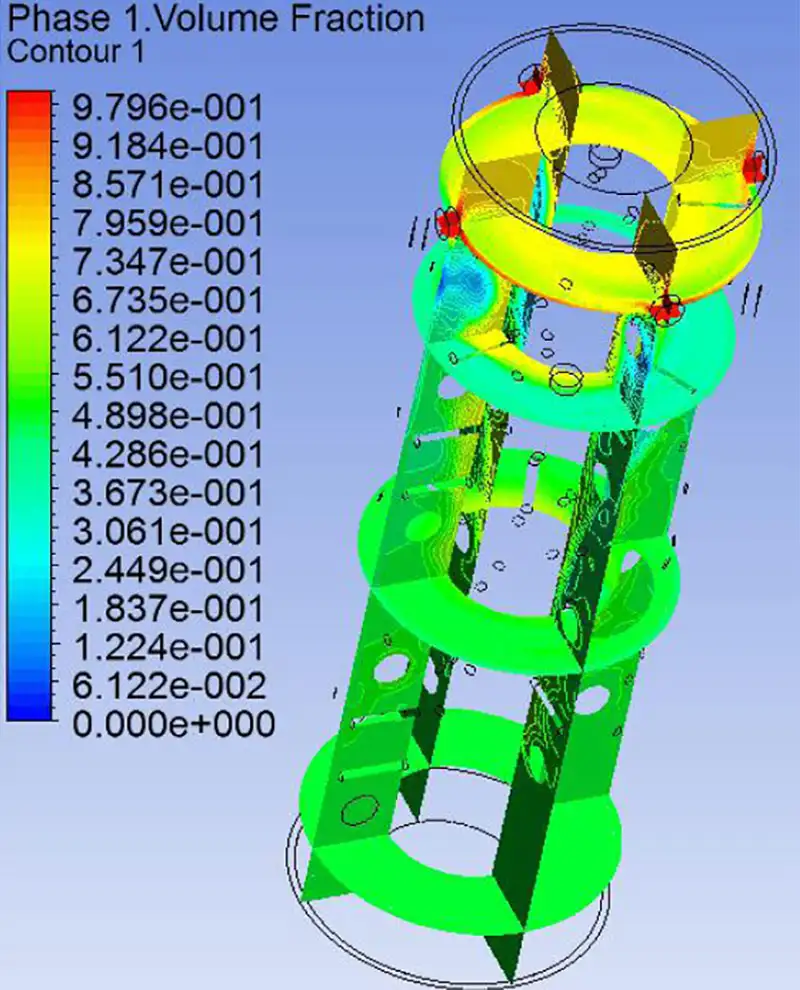
Volume Fraction Distribution Cloud Diagram of Materials
From the phase distribution cloud diagram, it can be observed that the mixing effect in the tubular reactor is excellent, ensuring thorough mixing of the reaction materials.
Previous page
Next page
Other News

