Application
Contact Us
Phone
Address
No. 60, Yungjing East Road, Liyuan Town, Tongzhou District, Beijing City, China
Nitration
Category:
Application
Nitration is the process of introducing a nitro group (NO₂) into an organic compound. Since the discovery of benzene nitration in 1834, this reaction has become a cornerstone in chemical synthesis. However, the nitration process requires the use of fuming nitric acid and concentrated sulfuric acid or glacial acetic acid (and sometimes acetic anhydride), and the significant exothermic nature of the reaction means that safety considerations are of paramount importance. For example, in Bretherick’s Handbook of Reactive Chemical Hazards, extensive discussions are presented on the incompatibilities of nitric acid with over 80 classes of reagents, along with the following cautionary statement: “If near stoichiometric composition, a homogeneous mixture of nitric acid and virtually any organic compound is a sensitive high-energy explosive.”
Continuous production can provide a valuable alternative to batch chemistry, as the reaction rate and temperature can be controlled by adjusting the flow rates of materials through the system.
Regulatory Requirements
Regulations in various countries promote the application of continuous automated equipment from the following perspectives:
Risk Control: Automation reduces the risk of human error and the potential for loss of control in batch processes.
Inherent Safety: Continuous processes maintain lower inventory levels of materials, making heat accumulation more manageable.
Compliance Enforcement: China and the European Union explicitly mandate the use of continuous processes through regulatory lists and directives. The United States and Japan indirectly drive the adoption by requiring comprehensive safety analyses.
Enterprises must align with local regulations and industry standards, prioritizing the implementation of continuous nitration processes. They should also integrate automated systems such as Distributed Control Systems (DCS) and Safety Instrumented Systems (SIS) to meet compliance requirements.
| Country | Regulation/Standard Name | Specific Requirement Clause | Type of Automated Equipment |
| United States | OSHA 29 CFR 1910.119(j)(4) | Automated Control Systems (Temperature and Pressure Control) | DCS |
| European Union | Annex II, Article 1.4 of the Seveso III Directive | Continuous Processes as a Best Available Technique (BAT) Option | DCS |
| European Union | Annex II, Article 1.4 of the Seveso III Directive | Continuous Processes as a Best Available Technique (BAT) Option | DCS |
| China | Clause 5.2.1 of GB/T 34325-2017 | Mandatory Continuous and Automated Processes | DCS + SIS Interlock |
| Japan | Article 28 of the Industrial Safety and Health Law | Automated or Remote Control Devices | Sealed Continuous Reactor |
Case
PMG led the effort to assist customers in maximizing profits from the limited space within existing facilities by producing the active ingredient in trifluralin.
The reaction primarily consists of two steps:
Step 1: Trifluorotoluene undergoes a single nitration to produce 4-chloro-3-nitrotrifluorotoluene. The reaction equation is as follows:
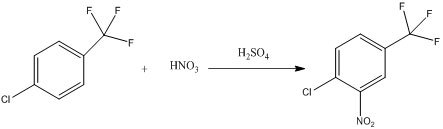
Step 2: Trifluorotoluene undergoes two nitrations to produce 4-chloro-3,5-dinitrotrifluorotoluene. In trifluorotoluene, the chlorine atom is an electron-donating group that activates the ortho position, while the trifluoromethyl group is an electron-withdrawing group that deactivates the ortho position. The nitro group is also an electron-withdrawing group that deactivates the ortho position. The selectivity of this reaction is relatively high and is primarily determined by the inherent structure of the molecule. The reaction equation is as follows:
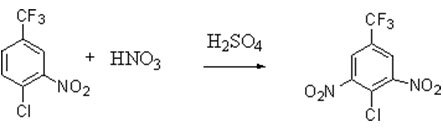
Based on the reaction data from the pilot study, the yield of the desired product was 96.55%, while the by-product yield was 2.54%. The experiment was conducted using a pilot-scale microchannel reactor. However, the residence time in the pilot study was relatively long. Given the high industrial throughput, the use of a microchannel reactor alone would result in a large pressure drop and excessively high investment costs. Therefore, a design was proposed that combines a microchannel reactor with a delay coil reactor. In this design, the residence time within the microchannel reactor is set at 40 seconds, while the residence time within the delay coil reactor is set at 120 seconds.

Photographs of the Industrial Production Equipment Site
Comparison of Production Efficiency Data
| Item | Batch Reactor | Microchannel Reactor |
| Personnel Required | 12 | 3 |
| Footprint | 230 m² | 6 m² |
| Reaction Time | 2 hours | 3 minutes |
| Conversion Rate | 90% | 92% |
| Safety Level | Low | High |
| Operation Mode | Batch | Continuous |
| Safety | Poor | High |
| Equipment Cost | Low | Slightly Lower |
| Characteristics | High pollution, low efficiency | Efficient, green, environmentally friendly |
Previous page
Next page
Other News

