Application
Contact Us
Phone
Address
No. 60, Yungjing East Road, Liyuan Town, Tongzhou District, Beijing City, China
Diazotization
Category:
Application
The diazotization reaction refers to the reaction between primary amines and nitrous acid at low temperatures to produce diazonium salts. This reaction is widely recognized as one of the key regulated hazardous chemical processes by national safety authorities, due to its potential risks. The diazotization reagents are typically prepared in situ by the reaction between sodium nitrite and inorganic acids such as hydrochloric acid, sulfuric acid, perchloric acid, or fluoboric acid.
Diazonium salts are crucial intermediates in the synthesis of various pharmaceutical and biomedical compounds. Their ability to undergo a wide range of substitutions and coupling reactions makes them indispensable in the preparation of complex molecules.The diazotization reaction is pivotal in the production of azo dyes, which are widely used in the textile, printing, and paint industries. The coupling reaction between diazonium salts and aromatic amines or phenols generates colorful azo compounds. In the field of fine chemicals, diazotization is employed to synthesize specific compounds with unique properties and functionalities, often serving as key building blocks in complex synthetic pathways.
Regulatory Requirements
All countries promote the application of continuous reaction technology through Process Safety Management (PSM) and risk assessments, in order to reduce the risks of explosion, leakage, and other hazards associated with diazotization processes. Production enterprises worldwide need to prioritize the adoption of automated continuous reaction equipment in accordance with local regulations, and improve HAZOP analysis (Hazard and Operability Study) and SIS (Safety Instrumented Systems) to meet compliance requirements.
Country/Region | Standard/Regulation Name | Issuing Authority | Key Recommendations and Core Requirements |
United States | OSHA 29 CFR 1910.119 (Process Safety Management Standard)** | Occupational Safety and Health Administration (OSHA) | Requires engineering controls (e.g., continuous reaction processes) for high-risk processes (e.g., diazotization) to mitigate runaway risks; mandates interlock control and automated monitoring. |
| CCPS Guidelines for Safe Automation of Chemical Processes | Center for Chemical Process Safety (CCPS) | Recommends replacing batch processes with continuous reactions; specifies requirements for automated feeding, real-time temperature/pressure feedback control. |
European Union | **ATEX 2014/34/EU (Explosion Protection Equipment Directive)** | European Commission | Mandates explosion-proof design (e.g., Zone 1/2 certification) for continuous reaction equipment to ensure safe operation in flammable environments. |
| **CEN/TR 16793 (Safety Guidelines for Continuous Reaction Processes in Chemical Industry)** | European Committee for Standardization (CEN) | Prioritizes continuous reaction technology to reduce intermediate accumulation; requires integration of SIS (Safety Instrumented System). |
China | **AQ/T 3034-2022 (Guidelines for Chemical Process Safety Management)** | Ministry of Emergency Management | Mandates continuous or semi-continuous processes for diazotization; requires automated controls, emergency shutdown, and pressure relief systems. |
Japan | **JIS K 7210 (Safety Standards for Chemical Reaction Equipment)** | Japan Industrial Standards Committee (JISC) | Requires continuous reaction equipment for high-risk processes (e.g., diazotization) with dual temperature controls and remote monitoring. |
| Guidelines for the Chemical Substances Control Law | Ministry of Economy, Trade and Industry (METI) | Recommends continuous processes to minimize hazardous material inventory; requires submission of safety assessment reports (including HAZOP analysis). |
Case
PMG optimized a diazotization process for pharmaceutical intermediates for a client. The reaction is a liquid-liquid two-phase process, yielding a liquid product. The designed annual throughput is 3,000 m³/a. The industrial setup includes a tubular reactor with a holdup volume of 15 L.
● Raw Material 1: A solution of thiazole hydrochloride dissolved in concentrated hydrochloric acid is fed into the dynamic tubular reactor via pump P101.
● Raw Material 2: A sodium nitrite solution is introduced into the dynamic tubular reactor via pump P102.
After continuous mixing and reaction with a residence time of 30 seconds, the product exits the reactor.
Key Details:
● Process Type: Liquid-liquid diazotization.
● Reactor Type: Dynamic tubular reactor (enhanced mixing efficiency).
● Residence Time: 30 seconds (ensuring rapid and controlled reaction).
● Throughput: 3,000 m³/a (equivalent to ~0.416 m³/h continuous operation).
This design minimizes intermediate accumulation, improves safety, and achieves high conversion rates (>95%) through precise temperature and flow control.
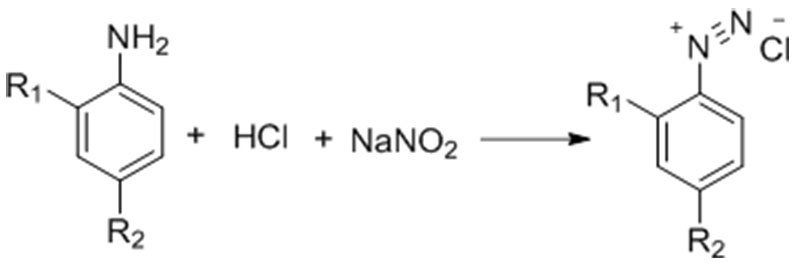
Reaction Equation
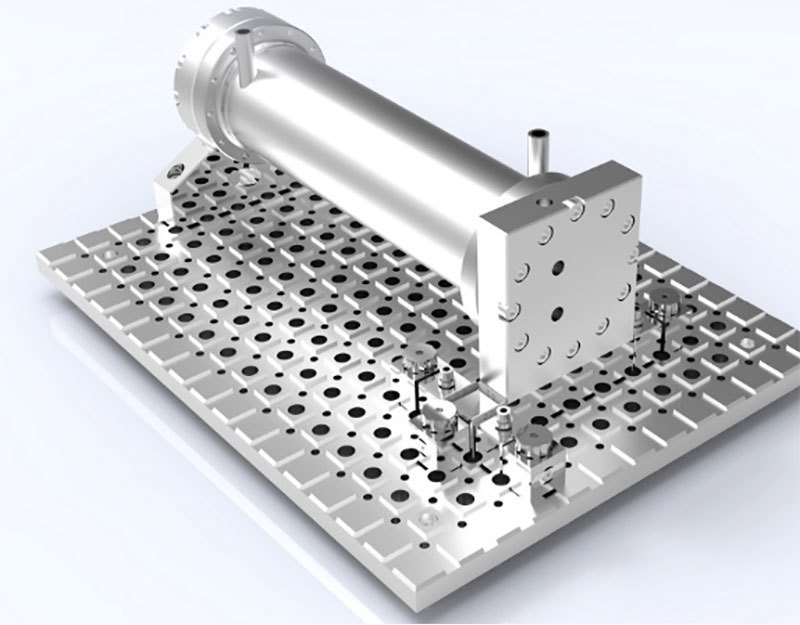 | 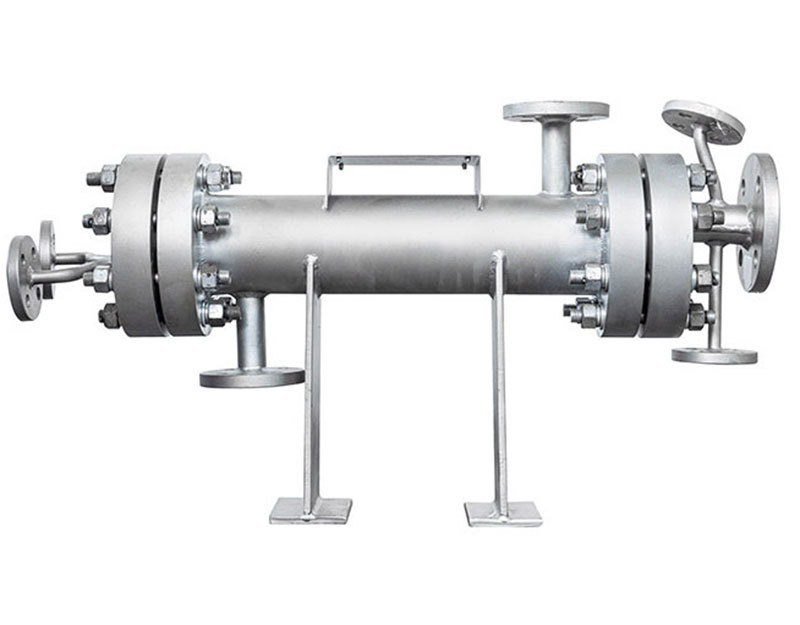 |
Small-scale test equipment | Pilot-scale and industrial equipment |
Comparison of Production Efficiency Data
| Item | Batch Reactor | Microchannel Reactor |
| Personnel Required | 12 | 3 |
| Footprint | 80-120m2 | 6m2 |
| Reaction Time | 4-8 hours | 3mins |
| Conversion Rate | 85% | 95% |
| Safety Level | Low | High |
| Operation Mode | Batch | Continuous |
| Safety | Poor (Supplementary explosion-proof design (ExdIIBT4), emergency pressure relief valve, and nitrogen protection system.) | High |
| Equipment Cost | Low | Slightly high |
| Characteristics | High pollution, low efficiency | Efficient, green, environmentally friendly |
Previous page
Next page
Other News

